|
Racial disparities seen in fistula use for dialysis patients - NephrologyNews.com |
 |
 |
|
Black and Hispanic patients will start hemodialysis with an arteriovenous fistula less frequently than white patients, according to a report published online by JAMA Surgery.
Mahmoud B. Malas, MD, MHS, of the Johns Hopkins Medical Institutions, Baltimore, and coauthors examined national trends in initial hemodialysis access with respect to race/ethnicity further divided by co-existing illnesses, nephrology care and medical insurance status.
Their study was a retrospective analysis of 396,075 patients with end-stage renal disease in the U.S. Renal Data System who started dialysis from 2006 through 2010. The main outcomes of the study were utilization rates of arteriovenous fistula (AVF), arteriovenous graft (AVG) and intravascular hemodialysis catheter (IHC). Most of the patients (55.4%) in the study were white, followed by 30.3% black patients and 14.3% Hispanic patients.
Read also: Monitor the dialysis access every day to make it last
The authors found that more white patients initiated hemodialysis with an AVF than black or Hispanic patients (18.3% vs. 15.5% and 14.6%, respectively), although black and Hispanic patients tended to be younger and had less coronary artery disease, chronic obstructive pulmonary disease and cancer than white patients with an AVF. Regardless of medical insurance status, both black and Hispanic patients started hemodialysis with an AVF less frequently than white patients. AVF utilization at initial hemodialysis also was lower among black patients and Hispanic patients compared with white patients among patients who had nephrology care for longer than one year.
The authors note it is possible black and Hispanic patients with chronic kidney disease may be progressing too quickly to ESRD to make AVFs a viable initial hemodialysis access option because AVFs generally take six to 12 weeks to mature and grow stronger.
"The racial/ethnic disparities in incident AVF access that we describe deserve elucidation. The high rates of catheter use despite national programs to reverse this trend is unacceptable. ... The sociocultural underpinnings of these disparities deserve investigation and redress to maximize the benefits of initiating hemodialysis via fistula in patients with ESRD [end-stage renal disease] irrespective of race/ethnicity," the study concludes.
"Their analysis of the U.S. Renal Data System contributes useful insight into racial/ethnic differences in arteriovenous fistula (AVF) utilization, accounting for patient comorbidities, insurance status and health care provider specialty, but the overall rates of AVF use (or more appropriately the lack of AVF use) at first hemodialysis are perhaps the more important and concerning finding," Laura A. Peterson, MD, MS, and Matthew A. Corriere, MD, MS, of the Wake Forest School of Medicinewrote in a related commentary. "Rates of AVF use at hemodialysis initiation were 18.3%, 15.5% and 14.6% among white, black and Hispanic patients, respectively. These results are especially sobering compared with the 2006 goals from the National Kidney Foundation, including prevalent functional AVF in more than 65 percent of patients and cuffed catheters in less than 10%.
... Given the mismatch between goals and current outcomes, the more appropriate quality improvement focus may be lowering the dismal overall catheter rates instead of a less than 5 percent difference in AVF rates between races/ethnicities."
|
|
Kaiser Opens New Rainbow Dialysis Center in Lahaina - Maui Now |
 |
 |
|
By Maui Now Staff
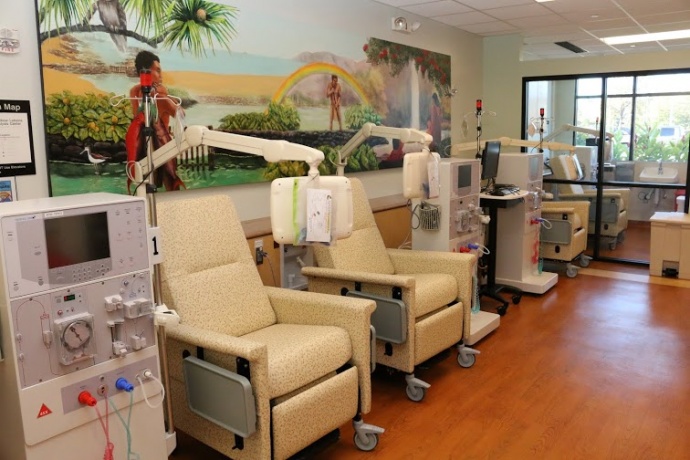
Kaiser Permanente held a blessing ceremony today for its newest facility on Maui. The Rainbow Dialysis Center, a wholly owned subsidiary of Kaiser Permanente Hawai?i, is located at Lahaina Gateway, just minutes from Kaiser‘s Lahaina Clinic. The new facility will begin treating patients in early May.
It is the second Rainbow Dialysis Center on Maui and has the capacity to treat approximately 18 kidney patients per day. The first Rainbow Dialysis Center location opened in Wailuku in June 2012.
The 2,000-square-foot facility is equipped with six dialysis stations, including an isolation unit to provide treatment for patients with contagious illnesses.
Kaiser representatives say the center will leverage services to improve the quality of care and provide patients treatment closer to home.
“Unfortunately, diabetes and resulting kidney disease are common health issues in Hawai?i, making dialysis an essential treatment for so many people,” said Geoffrey Sewell, MD, president and executive medical director of Hawai?i Permanente Medical Group in a press release statement. “We’re pleased to be able to provide patients on the Valley Isle with greater access to high-quality dialysis care at our new location in Lahaina.”
The Lahaina facility location will be led by medical director Alan Lau, MD, chief of nephrology at Kaiser Permanente; and will be managed by DaVita, one of the nation’s largest kidney care providers.
“Making quality health care convenient and affordable has always been our priority,” said Mary Ann Barnes, RN, president of Kaiser Permanente Hawai?i and Rainbow Dialysis in the announcement. “We are proud to be here today celebrating our second dialysis center in the Maui community, which will make treatments easier for the island’s dialysis patients,” she said.

Rudy Marilla, vice president of clinic operations; Mary Ann Barnes, RN, president of Kaiser Permanente Hawaii and Rainbow Dialysis; Kahu Earl Kukahiko; and Geoffrey Sewell, MD, president and executive medical director of Hawaii Permanente Medical Group, at the blessing of Lahaina Rainbow Dialysis Center on April 30. Courtesy photo.
Exterior of Lahaina Rainbow Dialysis Center. Courtesy photo.

Interior of Lahaina Rainbow Dialysis Center. Courtesy photo.
Recommend This Article <![CDATA[ .post_section_header, .post_section_header_noleft { width:735px; height:45px; background:url(http://mauinow.com/wp-content/themes/MauiNow3/images/post_section_header.png) no-repeat; line-height:35px; font-family:Arial; font-weight:bold; font-size:15px; color:#666; text-transform:uppercase; position:relative; left:-30px; } .post_section_header_noleft { left:-5px !important; } .posttags a { margin-left:5px; margin-right:5px; color:#333; } ]]><![CDATA[ ul#similarposts { list-style:none; width:100%; margin:0px; padding:0px; } ul#similarposts li { list-style:none; width:24%; display:inline-block; margin:3px; padding:0px; float:left; } ul#similarposts li .image { width:100%; height:100px; border:1px solid #ccc; overflow:hidden; margin-bottom:8px; } ul#similarposts li .image img { width:100%; min-height:100%; } ul#similarposts li a { text-decoration:none; font-weight:bold; line-height:16px; font-family:Arial; font-size:13px; text-align:Center; } ]]> You Might Also Like
|
|
|
Effingham Sheriff's Office hosting motorcycle ride for deputy - Savannah Morning News |
 |
 |
|
SYMPLICITY AF trial will evaluate renal denervation, pulmonary vein isolation ... - Healio |
 |
 |
|
Medtronic announced that enrollment has begun for the SYMPLICITY AF study, which will assess whether paroxysmal and persistent atrial fibrillation can be treated with a combination of renal denervation and pulmonary vein isolation.
Patients will be randomized to receive pulmonary vein isolation with a cardiac cryoablation system (Arctic Front Advance, Medtronic) alone, or pulmonary vein isolation plus renal denervation with a catheter (Symplicity Spyral, Medtronic) and a radiofrequency generator (Symplicity G3, Medtronic), according to a press release from the company. Renal denervation has previously been investigated as a treatment for hypertension.
All patients in the trial will receive an insertable cardiac monitor (Reveal LINQ, Medtronic) to detect and record recurrence of abnormal heart rhythms after randomization, according to the release.
“Hypertension is one of the most prevalent risk factors for developing AF, but we’ve seen that it is also potentially the most modifiable risk factor for halting the progression of the disease,” investigator Larry Chinitz, MD, director of the Heart Rhythm Center at NYU Langone Medical Center, said in the release. “As we continue to look for ways to prevent AF recurrence and improve outcomes for patients with AF, this trial may reveal a potential new treatment path for patients.”
The study will enroll up to 245 patients with paroxysmal or persistent AF from as many as 12 United States centers. Eligible patients will also have office-based systolic BP of 150 mm Hg or higher, despite receiving treatment with at least two antihypertensive therapies at the highest appropriate dose. Of those enrolled, 70 who meet all inclusion and no exclusion criteria will be randomized.
According to the release, the primary safety endpoint is comprised of events related to pulmonary vein isolation and renal denervation, and the primary efficacy endpoint is freedom from chronic treatment failure, defined as either AF of 30 seconds or longer or a required intervention for AF.
The renal denervation catheter and radiofrequency generator are not yet approved in the United States, and the cardiac cryoablation system is not yet approved for treatment of persistent AF, according to the release.
|
|
 Log in to explore the world's most comprehensive database of dialysis centres for free!
Log in to explore the world's most comprehensive database of dialysis centres for free!  Professional dialysis recruitment
Professional dialysis recruitment




 Kaiser Physicians Increase Pediatric Hospital Care on Maui
Kaiser Physicians Increase Pediatric Hospital Care on Maui Kaiser Employees Holding Six-Day Strike Statewide
Kaiser Employees Holding Six-Day Strike Statewide New Dialysis Unit Opens at Maui Memorial Medical Center
New Dialysis Unit Opens at Maui Memorial Medical Center
