|
Low Physical Function Not Tied to Muscle Mass - Renal and Urology News |
 |
 |
|
Phosphate Binders Cut Mortality, Up ESRD Risk - Renal and Urology News |
 |
 |
April 30, 2015
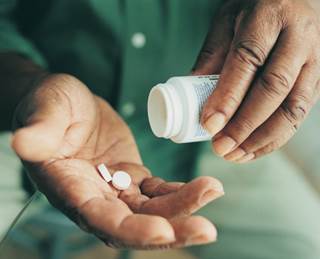
Compared with patients who did not use phosphate binders, those who did had a 15% decreased mortality risk and a nearly 3-fold increased risk of ESRD, the investigators reported.
Phosphate binder use was associated with lower mortality risk but a higher risk of end-stage renal disease (ESRD) among patients with chronic kidney disease (CKD) and high phosphorus levels, according to study findings presented at the National Kidney Foundation's 2015 Spring Clinical Meetings in Dallas.
In a retrospective longitudinal cohort study, Ardeshir Khosraviani, MD, and colleagues at Kaiser Permanente Southern California compared phosphate binder use and non-use among 3,026 non-dialysis CKD patients with hyperphosphatemia (phosphorus levels 5.5 mg/dL or higher). Compared with patients who did not use phosphate binders, those who did had a 15% decreased mortality risk and a nearly 3-fold increased risk of ESRD, the investigators reported.
“These findings underscore the need to better understand whether earlier phosphorus management may impact morbidity and mortality in advanced CKD,” the authors concluded in a poster presentation. Of the 3,026 subjects, 596 used binders and 2,430 did not. Study subjects had a mean age was 65.5 years; 49% were female, 49% were white, 24% were Hispanic, 17.3% were black, and 8.5% were Asian. The binder group had higher rates of diabetes, hypertension, and coronary artery disease.
“Our study raises the question of whether earlier management and control of hyperphosphatemia in the CKD population with binder therapy may improve patient survival in terms of mortality prior to and after [transition to ESRD],” Dr. Khosraviani told Renal & Urology News.
He said he and his colleagues believe their study adds to the literature on the topic because it looked at a real-world practice environment using a large and heterogeneous CKD population, and the results provide insights into CKD mineral-bone management strategies as patients transition to ESRD.
|
|
|
DaVita Healthcares (DVA) Q1 Earnings: What to Expect? - Zacks.com |
 |
 |
|
Rockwell Medical Inc.: Rockwell Medical Schedules First Quarter Earnings Call - The Wall Street Transcript |
 |
 |
|
Tickers: RMTI HNSN SURG WMGI
WIXOM, Mich., April 30, 2015 (GLOBE NEWSWIRE) -- Rockwell Medical, Inc. (Nasdaq:RMTI), a fully integrated biopharmaceutical company targeting end-stage renal disease (ESRD) and chronic kidney disease (CKD) with innovative products and services for the treatment of iron replacement, secondary hyperparathyroidism and hemodialysis, announced today that it will hold its quarterly conference call to discuss first quarter 2015 financial results on Thursday, May 7, 2015 at 4:30pm Eastern Time. This call is also being webcast and can be accessed at the Rockwell Medical Investor Relations web page. You can join this call on: About Rockwell Medical Rockwell Medical is a fully-integrated biopharmaceutical company targeting end-stage renal disease (ESRD) and chronic kidney disease (CKD) with innovative products and services for the treatment of iron replacement, secondary hyperparathyroidism and hemodialysis. Rockwell's recent FDA approved drug Triferic is indicated for iron replacement and maintenance of hemoglobin in hemodialysis patients. Triferic delivers iron to patients during their regular dialysis treatment, using dialysate as the delivery mechanism. In completed clinical trials, Triferic has demonstrated that it safely and effectively delivers sufficient iron to the bone marrow and maintains hemoglobin, without increasing iron stores (ferritin). Rockwell intends to market Triferic to hemodialysis patients in the U.S. dialysis market. Rockwell's FDA approved generic drug Calcitriol is for treating secondary hyperparathyroidism in dialysis patients. Calcitriol (active vitamin D) injection is indicated in the management of hypocalcemia in patients undergoing chronic renal dialysis. It has been shown to significantly reduce elevated parathyroid hormone levels. Reduction of PTH has been shown to result in an improvement in renal osteodystrophy. Rockwell intends to market Calcitriol to hemodialysis patients in the U.S. dialysis market. Rockwell is also an established manufacturer and leader in delivering high-quality hemodialysis concentrates/dialysates to dialysis providers and distributors in the U.S. and abroad. As one of the two major suppliers in the U.S., Rockwell's products are used to maintain human life by removing toxins and replacing critical nutrients in the dialysis patient's bloodstream. Rockwell has three manufacturing/distribution facilities located in the U.S. Rockwell's exclusive renal drug therapies support disease management initiatives to improve the quality of life and care of dialysis patients and are intended to deliver safe and effective therapy, while decreasing drug administration costs and improving patient convenience. Rockwell Medical is developing a pipeline of drug therapies, including extensions of Triferic for indications outside of hemodialysis. Please visit www.rockwellmed.com for more information. Certain statements in this press release constitute "forward-looking statements" within the meaning of the federal securities laws, including, but not limited to, Rockwell's intention to launch Calcitriol and Triferic following FDA approval. Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "predict," "forecast," "project," "plan", "intend" or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Rockwell Medical believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including without limitation those set forth in Rockwell Medical'sSEC filings. Thus, actual results could be materially different. Rockwell Medical expressly disclaims any obligation to update or alter statements whether as a result of new information, future events or otherwise, except as required by law. Triferic? is a trademark of Rockwell Medical, Inc.
CONTACT: Michael Rice, Investor Relations; 646-597-6979 Source: Rockwell Medical, Inc. News Provided by Acquire Media
|
|
 Log in to explore the world's most comprehensive database of dialysis centres for free!
Log in to explore the world's most comprehensive database of dialysis centres for free!  Professional dialysis recruitment
Professional dialysis recruitment



